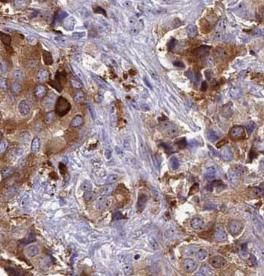
anti- VPRBP antibodyСпецификация| Clonality | polyclonal | | Host | Rabbit | | Specificity | Human, Mouse, Rat | | Tested Application | ELISA, WB, IHC, IP, FC | | Delivery Time | 2 to 4 working days | | Isotype | IgG | | Form | liquid | | Purification | Immunogen affinity purified | | Purity | ≥95% as determined by SDS-PAGE | | Uniprot ID | Q9Y4B6 | | Gene ID | | | Calculated MW | 11 kDa | | Ссылка на страницу на сайте производителя | ссылка | | Инструкция | PDF | | Storage | PBS with 0.02% sodium azide and 50% glycerol pH 7.3,-20℃ for 12 months(Avoid repeated freeze / thaw cycles.) | | Background | Acts both as a substrate recognition component of E3 ubiquitin-protein ligase complexes and as an atypical serine/threonine-protein kinase, playing key roles in various processes such as cell cycle, telomerase regulation and histone modification. Probable substrate-specific adapter of a DCX(DDB1-CUL4-X-box) E3 ubiquitin-protein ligase complex, named CUL4A-RBX1-DDB1-DCAF1/VPRBP complex, which mediates ubiquitination and proteasome-dependent degradation of proteins such as NF2. Involved in the turnover of methylated proteins: recognizes and binds methylated proteins via its chromo domain, leading to ubiquitination of target proteins by the RBX1-DDB1-DCAF1/VPRBP complex(PubMed:23063525). The CUL4A-RBX1-DDB1-DCAF1/VPRBP complex is also involved in B-cell development: VPRBP is recruited by RAG1 to ubiquitinate proteins, leading to limit error-prone repair during V(D)J recombination. Also part of the EDVP complex, an E3 ligase complex that mediates ubiquitination of proteins such as TERT, leading to TERT degradation and telomerase inhibition(PubMed:23362280). Also acts as an atypical serine/threonine-protein kinase that specifically mediates phosphorylation of 'Thr-120' of histone H2A(H2AT120ph) in a nucleosomal context, thereby repressing transcription. H2AT120ph is present in the regulatory region of many tumor suppresor genes, down-regulates their transcription and is present at high level in a number of tumors(PubMed:24140421). Involved in JNK-mediated apoptosis during cell competition process via its interaction with LLGL1 and LLGL2(PubMed:20644714). In case of infection by HIV-1 virus, it is recruited by HIV-1 Vpr in order to hijack the CUL4A-RBX1-DDB1-DCAF1/VPRBP function leading to arrest the cell cycle in G2 phase, and also to protect the viral protein from proteasomal degradation by another E3 ubiquitin ligase. The HIV-1 Vpr protein hijacks the CUL4A-RBX1-DDB1-DCAF1/VPRBP complex to promote ubiquitination and degradation of proteins such as TERT and ZIP/ZGPAT. In case of infection by HIV-2 virus, it is recruited by HIV-2 Vpx in order to hijack the CUL4A-RBX1-DDB1-DCAF1/VPRBP function leading to enhanced efficiency of macrophage infection and promotion of the replication of cognate primate lentiviruses in cells of monocyte/macrophage lineage. | | Immunogen | Vpr(HIV-1) binding protein | | Synonyms | DCAF1, HIV 1 Vpr binding protein, KIAA0800, Protein VPRBP, RIP, Vpr(HIV 1) binding protein, Vpr interacting protein, VPRBP | | Recommended dilution | WB: 1:500-1:5000; IP: 1:500-1:5000; IHC: 1:50-1:500 | Immunohistochemistry of paraffin-embedded human prostate cancer tissue slide using FNab09424(VPRBP Antibody) at dilution of 1:50
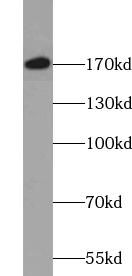
 | HeLa cells were subjected to SDS PAGE followed by western blot with FNab09424(VPRBP antibody) at dilution of 1:500
 | | | |
Информация для заказа| Область использования: | Производство: | Fine Biotech | | Метод: | Антитела | | Объем: | 100µg | | Кат. номер: | FNab09424 | | Цена (с НДС 20%): | по запросу | В корзину  |  Наименование: anti- VPRBP antibody. Наименование: anti- VPRBP antibody.
Примечание: |
|
